
Three main models of how to elicit meaningful
stakeholder participation in CPG development exist: (1)
direct membership of the panel; (2) evaluation of evidence
outside of panel meetings (eg, via the formation of an expert
patient guideline group, via a one-off meeting, or via a series
of CPG workshops with stakeholders); and (3) having a
‘‘skilled member’’ to speak for the wider patient/stakehold-
er group (eg, the director of a charity)
[6]. The ‘‘skilled
member’’ model has been favoured in practice
[2], but this
raises the question of how this can then transcend
individual bias, national boundaries, cultures, differences
in the process of health care, and how it is to be funded.
Finally, there is the question of how the input of each panel
member is assessed, in parallel with evaluation of the
guidelines themselves, and the costs/benefits of different
stakeholder engagement. Measurable outcomes (eg, adher-
ence to CPGs, adherence to treatment, costs of care) will
define CPG efficacy, together with qualitative outcomes
such as patient-centred care and shared-decision making.
1.
Proposed model
The core principles of CPG development are transparency,
accountability, and harmonisation of patient care based on
the best available scientific evidence. We propose a feasible
model, currently being operationalised by the European
Association of Urology (EAU), for CPGs to serve key
stakeholders, which will also benefit the implementation
of guidelines
( Fig. 1 ).
First, an effective panel must be redefined. Historically,
panels have grown organically from the network of the
appointed chair/vice-chair. To professionalise this process,
Box 1. Checklist to achieve multidisciplinary stake-
holders on a clinical practice guideline panel.
U
Define the remit of the panel and the roles of each
place on the panel; specify rules for the process
U
Identify key stakeholder functions, potential mem-
bers:
medical specialists
junior associates able to generate systematic reviews
for recommendations
non-medical health professionals (nursing, para-
medical, health economist)
patient representation (determine global/interna-
tional/national)
healthcare funders
charitable organisations
U
Interview all potential members for skill-based func-
tion on panel, impartiality, transparency, and ability
to commit to a term and workload
U
Assess conflicts of interest and ensure that panel
members do not vote on or influence any issues
where they are conflicted
U
Train all panel members in evidence-based medicine
methodologies
U
Define outreach outcomes per member (eg for the
patient representative feedback from the communi-
ty, priority setting) to generate feedback cycle
U
Evaluate member function annually, outcomes de-
livered
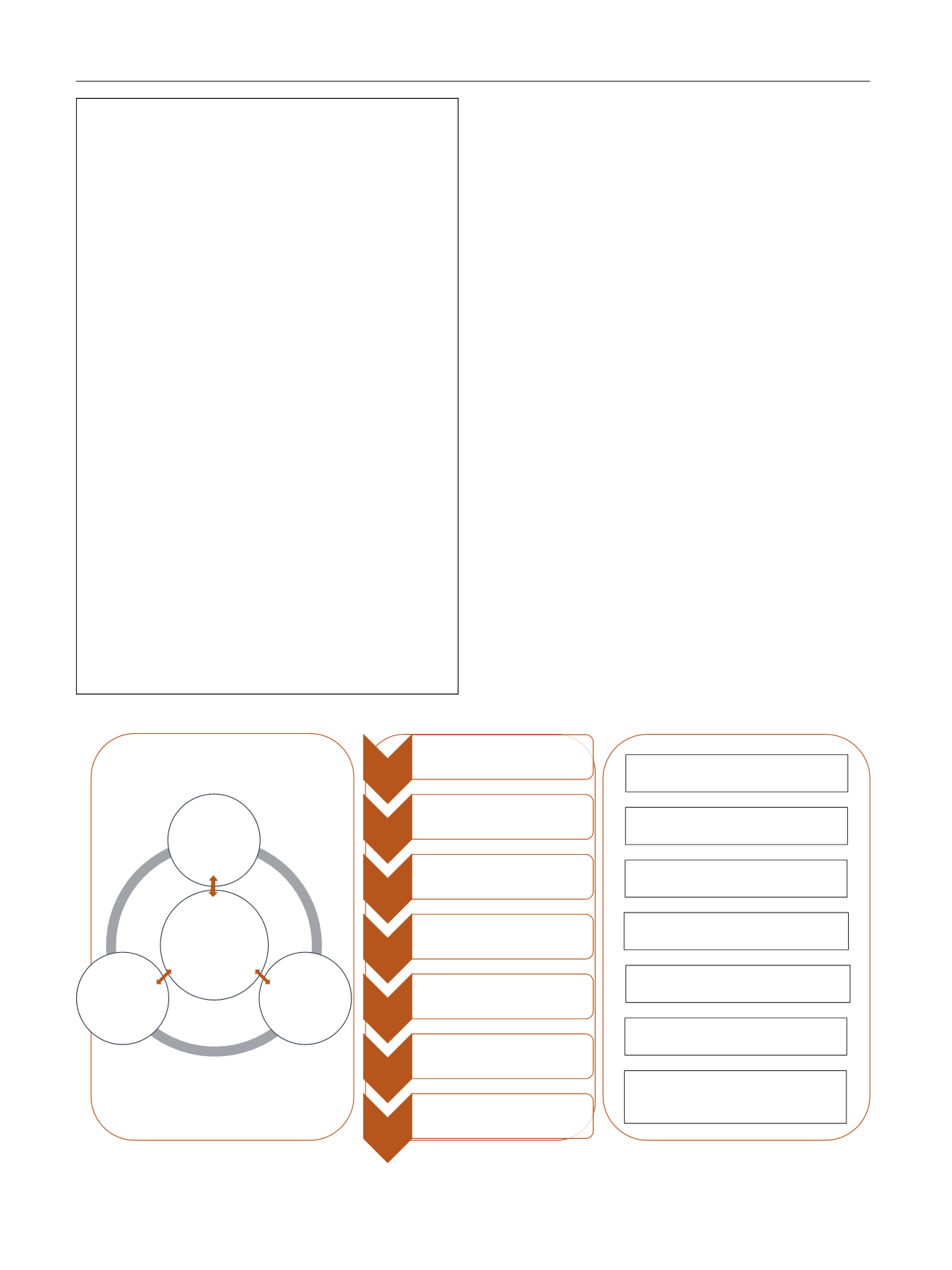
[(Fig._1)TD$FIG]
Structure
Process
OpportuniƟes for Involvement
Research
PrioriƟsaƟon
•Panel or paƟent groups propose topics
•PrioriƟse the topics against criteria
•ConsultaƟon exercise
PICO
•Answerable quesƟons formulated
Choose
outcomes
•COMET database checked for core outcome set
•If a COS does not exist, outcomes should be
chosen by panel but paƟent groups consulted
•A COS should be developed
SystemaƟc
review
•The associates undertake the SR under the
supervision of the panel and the methods
commiƩee
GRADE the
evidence
•The quality of evidence is assessed by the panel
and recommendaƟons made
Publish
guidance
•The CPG is acƟvely disseminated to target
audiences
Update
•The CPGs should be updated annually
IdenƟfying and seƫng prioriƟes for
guidelines and recommendaƟons
Establishing the scope of the guidelines
or recommendaƟons
Defining the types of outcomes we
can/should measure
Reviewing the evidence used in
guidelines and recommendaƟons
Co-developing the draŌ guidelines and
recommendaƟons
ParƟcipaƟng in the disseminaƟon of
guidelines and recommendaƟons
FacilitaƟng research on gaps in the
evidence based on guidelines and
recommendaƟons
Guideline
development - key
stakeholders e.g.
clinical expert,
expert paƟent,
methodologist,
clinical associates,
editorial staff
Key
internaƟonal
health and care
organisaƟons
Key naƟonal
health and care
organisaƟons
Wider
consultaƟon e.g.
policy makers,
funding bodies
Fig. 1 – Proposed framework for structure and implementation of stakeholder involvement in clinical practice guidelines (CPGs).
E U R O P E A N U R O L O G Y 7 2 ( 2 0 1 7 ) 1 6 1 – 1 6 3
162
















